How To Draw Pi Molecular Orbital Diagrams
8 - Drawing Molecular Orbital Diagrams

Abstruse (TL;DR)
Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals grade using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are different, these molecular orbital diagrams will look incredibly different. Regardless, consideration of the symmetry between atoms, how the orbitals mix and the electronegativities of all atoms are necessary in drawing molecular orbital diagrams. This lesson walks through those considerations.
———
The waves of electrons within diminutive orbitals (left and right) forming a pi molecular orbital (top, middle) through subversive interference and a sigma molecular orbital (top, bottom) through effective interference.
Organization of electrons within molecular orbitals is quite a bit more than involved than diminutive or hybridized orbitals. In fact, it'south that complexity that makes it so that the simpler valence bond (VB) theory is the preferred way to teach chemic bonding. Still, equally we discussed, molecular orbital (MO) theory introduces a level of detail missing from valence bond theory.
To make sense of the complexities introduced by antibonding, we build molecular orbital diagrams. These, however, as well accept their own depth; diagrams like these change according to if yous're working with homonuclear molecules - molecules made of one element - or heteronuclear molecules - molecules fabricated of unlike elements. Yes, readers, unfortunately, this is going to be similar to the functional groups section in its depth. So allow'southward start slowly and, outset, describe what these diagrams are at their most basic.
Molecular Orbital Diagram
As we've established (link to last lesson), bonding and antibonding interactions are the key to molecular orbital theory. In fact, the idea of molecular orbitals, the distribution of electrons across a molecule, arises from how electrons distribute themselves energetically.
Call back, antibonding occurs when electrons experience a force that prevents them from engaging in bonding. This would merely happen if electrons were distributed in such a way that acquired them to destructively interfere with each other. In that example, y'all might imagine that the energy of the electrons would increase, and, y'all would be right - as electrons near each other, energy increases (one might call that a very fundamental lesson).
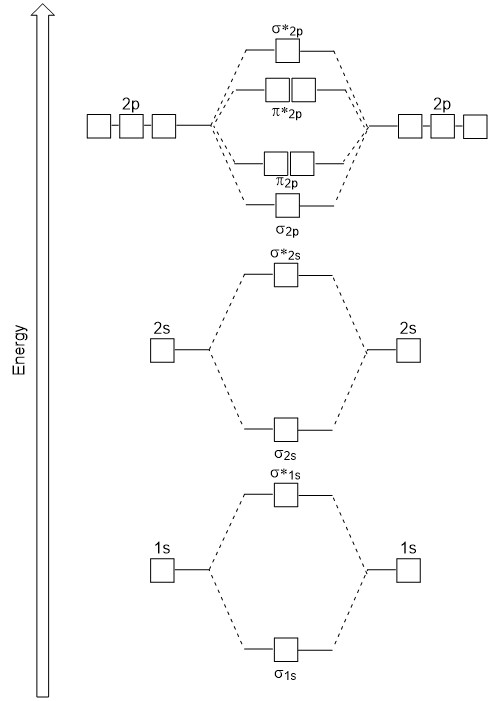
Given that some electrons exist in these "antibonding regions" and others exist in the "bonding regions", we tin can redefine molecular orbitals as "the distribution of antibonding and bonding electrons across a molecule".
This, of course, needs to be represented somehow - just similar we've modeled atomic orbitals and hybridized orbitals. That is where the molecular orbital diagram (Modernistic) comes in.
At start glance, these diagrams looks terrifying. However, nosotros tin can rely on a lot of what we already know to help us understand what's going on here.
Parts of the MOD
First, as we've learned, the more electrons that an atom has, the higher its cumulative energy. MODs reflect this by having the lowest atomic orbitals at the lesser of the diagram and the highest orbitals toward the peak.
You'll besides notice that each module in a molecular orbital diagram has two sides and a middle department. Each of the two sides stand for the atomic orbitals of each cantlet involved in the bonding, while the center department represents the molecular orbital formed as the diminutive orbitals combine. As you lot would imagine, the meat (and the complexity) is in the middle of this sandwich.

Check Your Electrons
You can e'er check if you've written your diagrams correctly past adding the diminutive orbitals together and seeing if they equal the molecular orbitals. Yous tin also add the number of each orbital type'south electrons; they should as well exist equal.
Organizing Molecular Orbitals
These diagram reveal only how complementary the MO theory and VB theory are through the presence of the sigma (σ) and pi (π) symbols. In molecular orbital theory, sigma and pi mean the aforementioned thing that they meant in valence bond theory, with a few exceptions.
Firstly, nosotros now add together our newly caused antibonding molecular orbitals. There are now four types of orbitals to concern ourselves with: (ane) sigma bonding, (two) sigma antibonding, (3) pi bonding, and (4) pi antibonding.
The style these bonds are placed on whatever molecular orbital diagram is according to how the atomic orbitals that brand the MOs mix. In that mixing, in that location are 2 factors to consider: (1) atomic symmetry and (2) mixing.

Symmetry
As a bail occurs, a bond or internuclear axis - the line that connects the nuclei of ii bonded atoms - forms.
Internuclear axes aren't new to us - they've existed e'er since we started speaking about bonds. Further, in introducing sigma and pi bonds, we've already begun the conversation about symmetry. Information technology is symmetry that defines what sigma and pi mean with respect to molecular orbital theory.

In the diatomic hydrogen molecule to the right, if i were to rotate the two overlapped atomic orbitals about their internuclear centrality and no modify results — constructively interfering (in-stage) atomic orbitals continued to constructively interfere and vice versa for destructively interfering (out-of-phase) diminutive orbitals — you lot would have an example of sigma bonding or antibonding molecular orbital (according to the phase). We call this symmetry.

Paradigm from Dr. Adam via Professor Adam Teaches
An example of 1s diminutive orbitals combining to form: (top) a sigma bonding molecular orbital and (bottom) a sigma antibonding molecular orbital. Take annotation that, in both, if yous rotated the molecular orbital, there are no changes in stage, making them sigma.
Pi molecular orbitals, as you might have already guessed, are the opposite. They form when there is a alter in phase after rotating ii overlapped atomic orbitals about the internuclear axis. Nosotros call this asymmetry.

Image from Dr. Adam via Professor Adam Teaches
An example of the pi antibonding orbital, formed from out-of-phase 2p orbitals. Note how, after rotating, the outcome is different - a phase change.
| | | | |||
|---|---|---|---|---|---|
| Sigma | Symmetry | | |||
| Pi | Asymmetry | | |||
In other words, sigma molecular orbitals are the effect of symmetric interactions between atomic orbitals, while pi molecular orbitals are the upshot of asymmetric interactions.

Advanced Symmetries
When we leave the p orbitals and motility into the d and f orbitals, we proceeds not just sigma and pi molecular orbitals, just also delta (δ) and, potentially, phi (φ) molecular orbitals besides.
Mixing
With terms like "overlap" and "combine", yous might be confused as to why I'one thousand singling out "mixing". Mixing is a little more circuitous.
"Mixing" is a theoretical explanation for how sure atoms interact to course molecular orbitals. This mixing is similar to hybridizing, wherein diminutive orbitals change to stabilize a molecular orbital's free energy distribution. The main difference is that hybridization occurs independently of any other atomic orbitals, while mixing happens between two or, sometimes, more atomic orbitals.
With that said, how does mixing happen? To explain, let'due south put 2 nitrogen atoms together and explain our get-go type of molecular orbital - homonuclear molecular orbitals.
Homonuclear Molecular Orbitals
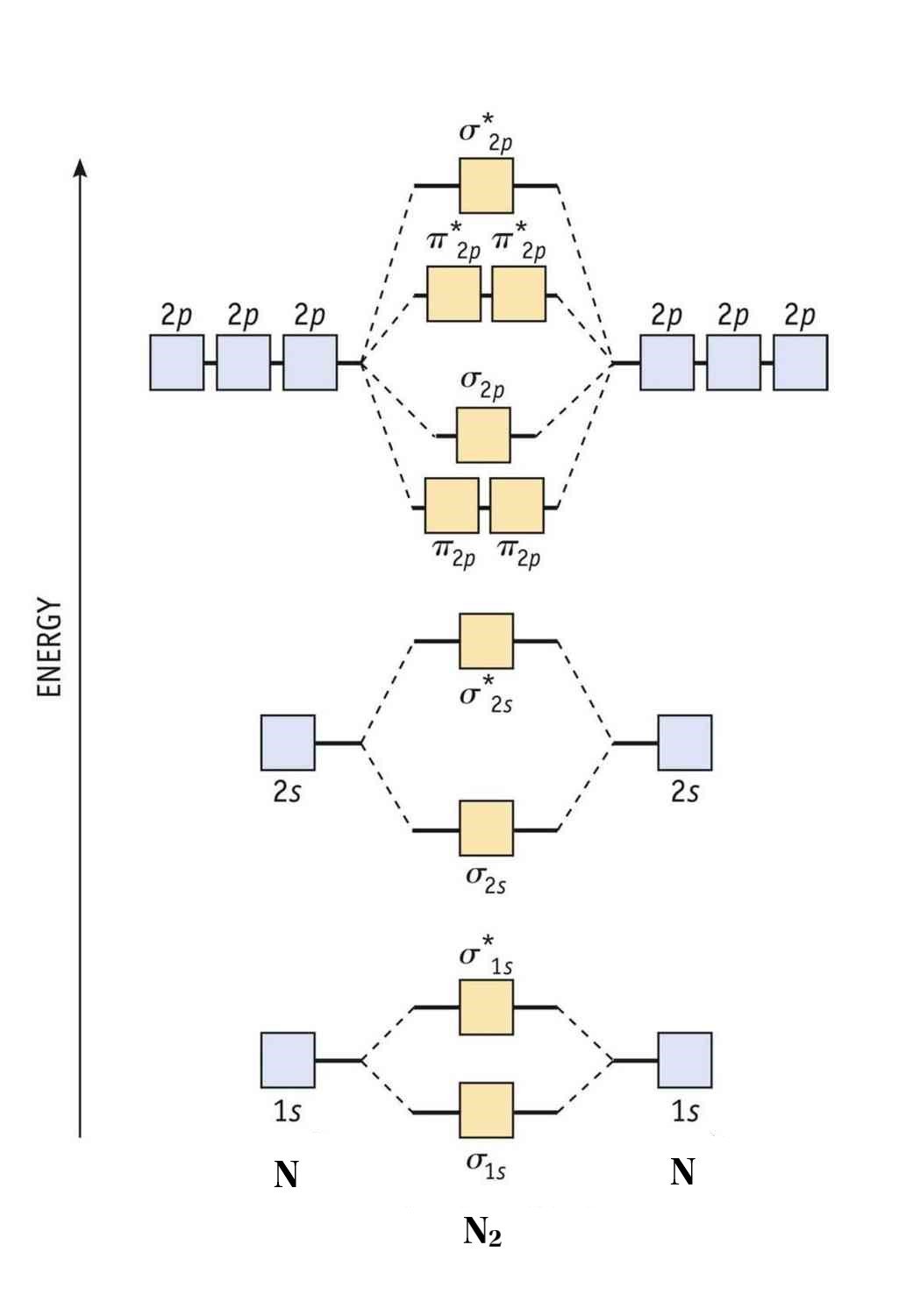
Epitome via Bartleby
Molecular orbital diagram of diatomic nitrogen.
Homonuclear molecular orbitals are formed betwixt ii elements that are the same, meaning that they are naturally symmetrical and volition perfectly overlap.
However, earlier nosotros fill out this diagram, compare this MOD to the one above, particularly in the 2p region. They're pretty like, except with their sigma and pi bonding molecular orbitals. That'south because diatomic nitrogen exhibits the effects of mixing, which lowers the energy of the sigma 2p MO to below that of the pi 2p MO. Just how?
To explain, we have to go back to a classic - effective nuclear accuse. The effective charge determines how many reactive electrons tin can be drawn to an atom. Nosotros besides know that the effective accuse increases every bit the periodic group increases, or equally you go further to the right on the periodic table.
Simply something happens to the orbitals the higher the constructive charge gets - they shrink. Simply put, as electrons are more attracted to the nucleus in atoms with higher effective charges, the sizes of the orbitals go smaller and tighter.
In terms of atomic energy, every bit orbital sizes decrease, they commencement to become more than uniform - that is, the energies of college orbitals begin to come up closer to the energy of lower ones. Further, the energy of lower orbitals also rises. It is this interaction betwixt orbitals that gives "mixing" its name.
With that said, the specific mixing that occurs in diatomic nitrogen is called sp mixing, which is mixing between the sigma 2s and 2p MOs.
Oxygen, as we know, has the configuration [He]2s22p3. That means that ane oxygen atom has 2 core electrons and 5 valence electrons, for a total of fourteen electrons, when factoring both nitrogen atoms - the first display of differences between molecular orbital theory and valence bail theory.
To fill the diagram, outset, we fill each side of the diagram with the electrons according to nitrogen'southward electron configuration - [He]2s22p3. Next, we fill up the center section with the molecular orbital'southward electron configuration using Hund's Rules, simply as we do with atomic orbitals. We fill each beat with two electrons earlier moving to the side by side, and, for the orbitals with multiple subshells, like the 2p beat out, we fill up each subshell with one electron before completing the subshell with the 2nd.


Limits of sp Mixing
In the second catamenia, simply lithium to nitrogen undergo sp mixing, when i is bonded to itself. Oxygen and fluoride practice non.
What about diatomic oxygen?
Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram similar the starting time in this article. This is considering, every bit more electrons are added to a organization, the higher the energy becomes, due to their electrostatic repulsion. If the free energy of the 2s and 2p orbitals are besides far apart, mixing won't occur. Therefore, you get a molecular orbital diagram that's what you lot expect - the sigma molecular orbital being lower energy than the pi molecular orbital.
With that change in listen, filling this MOD is the aforementioned as filling diatomic nitrogen's:

Notation the swapped sigma and pi 2p MOs.
Homonuclear MODs show quite a bit of the ingenuity of this bonding theory considering it's every bit though it is a natural continuation of the atomic orbitals we know and love. Heteronuclear MODs, on the other hand, are a lot more involved.
Heteronuclear Molecular Orbitals

Carbon Monoxide, composed of a carbon atom (cerise) and oxygen atom (grey)
Carbon Monoxide. The reason yous desire to be wary of heating sources, and one of those no-no chemicals when it comes to sustaining human life.
This molecule is interesting. Despite neither carbon or oxygen having stability on their ain, from the point of their molecular orbitals, it is a stable molecule. But how is its electrons organized from the perspective of a molecular orbital diagram?
For heteronuclear molecules like these, the first consideration is electronegativity. Electronegativity, if you think, is the tendency of an atom to pull electrons toward its nucleus. Nosotros know that the closer electrons are to the nucleus, the more stable the atom is (the reason that cadre electrons aren't reactive).
This means that electronegative atoms have lower atomic free energy than electropositive atoms. This results in a Modernistic that has a "slanted" course.

Carbon monoxide's Modernistic, not including the core, nonbonding electrons within each cantlet's 1s orbital.
The electronegativity likewise influences the grapheme of these molecular orbitals. Imagine it this way - oxygen is ravenously cartoon all nearby electrons closer. Carbon, as passive every bit it is, is alright with existence dragged along with its electrons. In this state of affairs, which cantlet influences the shape of the molecular orbitals the near? And in what ways?
Well, since molecular orbitals are composed of both bonding and antibonding orbitals, we can say that oxygen influences the bonding aspect the most since its electronegativity is a constructive force for bonding. Further, if oxygen's atomic orbitals are influencing the bonding molecular orbitals, then carbon's atomic orbitals would influence the antibonding molecular orbitals.
This makes the position of the molecular orbitals a little clearer. Can you see how the bonding and antibonding orbitals skew toward either carbon or oxygen?
This demonstrates a valuable rule within MO theory: when atomic orbitals do non take the same energy levels, the molecular orbital that'southward formed is well-nigh similar to the atomic orbital that is closest to it in energy.
Mixing also occurs between heteronuclear MOs, if the free energy between the atoms is close enough. This is the case in carbon monoxide, in which sp mixing occurs.
Sp mixing is a bit different in heteronuclear MOs, but the concept is the same. Molecular orbitals that are shut enough in energy volition mix, but, due to the differences in electronegativity, the symmetries of each atom'southward orbitals become more important. Fortunately, our molecular orbital diagram higher up uses dotted lines to demonstrate which atomic orbitals mix to course which molecular orbitals.
Even more than fortunate is, one time you lot've determined how to draw the molecular orbital diagram, the rules for filling MO diagrams remain the same every bit ever. Our filled MO is:

Loose Ends
If you fabricated it through that explanation of molecular orbital theory, you've learned something that even college students struggle with - very well done. But, going through it, you lot might take some questions.
Outset, the d orbital. Since it's got 5 subshells, there'south a lot less likelihood that there is symmetry between them. That means, there are only a few combinations of atomic orbitals that may work to grade molecular orbitals. That is also why educators prefer to teach MO theory using the elements in the first and 2d menstruation - the due south and p orbital elements.
But, perhaps more importantly, in the last lesson, I referenced that the idea of valence bail theory being insufficient, as the idea of electrons being solely located in between atoms is unrealistic. These systems, also known as localized systems, make understanding bonding very simple (hence why bonding is taught with the valence bond theory). However, we needed molecular orbital theory - a delocalized system, where electrons are distributed all throughout a molecule instead of just i area - to explicate the more realistic scenario. This lack of an caption for delocalization was i of the biggest weaknesses of valence bail theory.
However, my mentioning of its weakness doesn't hateful that valence bond theory doesn't have an answer at all. With our understanding of delocalized systems in the molecular orbital theory, we'll discuss delocalization within the valence bail theory in the next lesson.
Source: https://www.fluxsci.com/flux/mods
Posted by: nolinwounamed1983.blogspot.com


0 Response to "How To Draw Pi Molecular Orbital Diagrams"
Post a Comment